Retinol, a form of Vitamin A is arguably one of the most loved and widely used skincare ingredients on the market with products that come in a variety of formulations, textures and concentrations marketed by big beauty giants as one stop solutions to aging, fine lines and wrinkles and frequently prescribed by derms for acne. While it does have scientifically proven results, chances are that it might be overconsumed due to that very reason, or so the Scientific Committee of Consumer Safety (SCCS) has found.

It looked into the possible worst case scenarios of consuming Vitamin A through various sources like supplements, food, medicines and skincare together in combination, noting what side effects it might have on the body and found that extreme exposure can cause complications as serious as liver damage, headaches, nausea, bone thinning, and birth defects. In light of these findings the EU has released new retinol legislation for the percentage of retinol allowed to be used in over the counter skin care, making 0.3% the limit for facial products and 0.05% for body care since the latter covers larger surface area on the skin.
It is already common knowledge in the skincare and beauty space that some ingredients like retinol and its derivatives like retinaldehyde and retinoic acid are not pregnancy-safe and should be avoided if you are expecting or breastfeeding since there is not much data and studies on its effects on the mother and child due to ethical reasons. This information therefore comes as a surprise since all beauty lovers and influencers are using and promoting retinol, brands are coming up with new formulations and there is more awareness about ingredient based products where the ingredient sells the range.
Skinfluencers and even internet dermats are seen using potent actives, harsh chemical peels and procedures which supposedly miraculously improve their skin, misleading audiences. While professionally done procedures can be beneficial to some extent, Indian skin and other skin tones which fall on the darker end of the Fitzpatrick Scale are more prone to irritation from artificial procedures and ingredients than white skin. Keep in mind most skincare procedures, products and brands cater to lighter skin tones and are almost always designed and tested for white skin consumers. It’s crucial to check all ingredients in the inky list since many times brands only mention the marketed ingredients rather than the full list. Read the list correctly, understanding that the first ingredient (generally water) is the highest in concentration and it goes in descending order with the last ingredient being the lowest in concentration. Content creators boast of fancy clinics and expensive brands which sometimes don’t even have permits and certifications yet their loyal fanbase falls trap to it resulting in skin diseases and irritation. The false advertisement epidemic within the Indian skincare industry is dangerous also because healing can take so much time and resources once there are conditions like hyperpigmentation, dermatitis, acne developing from skin irritation and even flesh eating skin diseases.
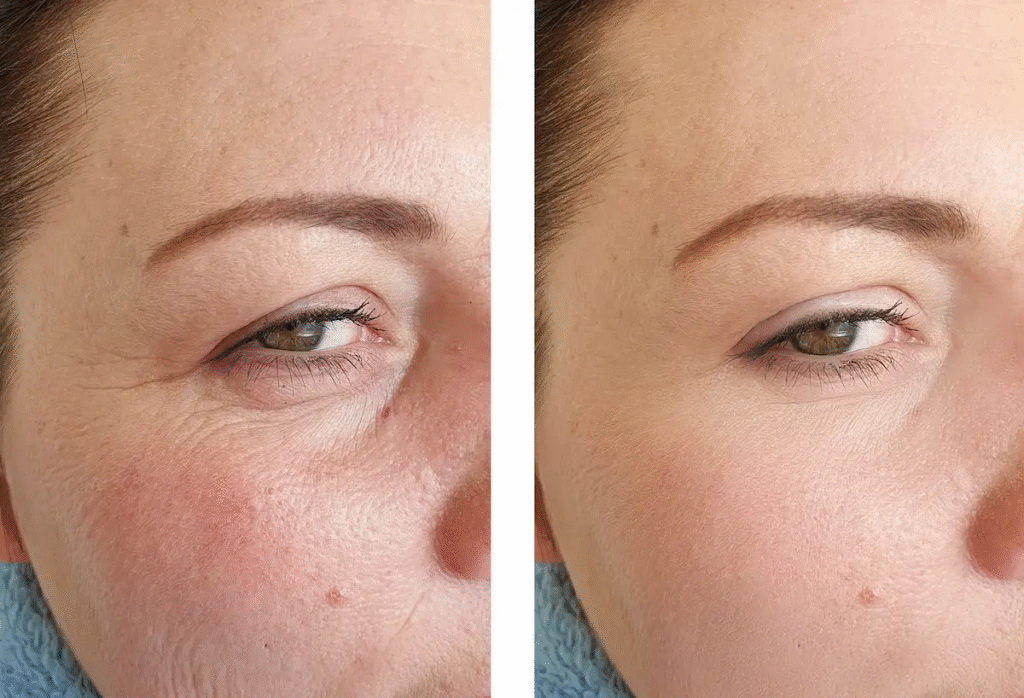
The situation hints at a balanced approach to beauty where you focus on quality rather than quantity since retinol as low as 0.01% shows a significant reduction in signs of aging. Brands generally pump up the percentages to show better promise to the consumers, however higher retinol concentrations can lead to skin irritation, dryness, flaking and for darker skin tones, hyperpigmentation. Some brands even mislead consumers into thinking that encapsulated retinol of 4% delivers at face value when it actually has only 0.2% of it overall mixed with 3.8% stabilizers.
Though the risk is slight, brands have been given time of three years to reformulate their current products. Since the old products on the market are not altogether harmful, they will be removed later by 1 May 2027 while new ones after 1st November 2025 will have to adhere to the latest guidelines. Moreover, transparency must be maintained on all retinol products clearly stating ‘Contains vitamin A-related compounds, which contribute to your daily intake of vitamin A’ to eliminate further risk.
Health Canada has also suggested a revision on its regulated retinol concentration and its esters in certain products like body lotions to stay under the tolerable Upper Intake levels of Vitamin A. Retinal is also being restricted for its therapeutic properties and risk of skin irritation. The ingredient is currently listed in Health Canada’s Natural Health Products Ingredients Database with a limit of 0.05% for use as a non-medicinal ingredient in creams.
The FDA too seems concerned about drug claims made for cosmetics and skin care marketed as anti aging creams but alter the normal functioning of the skin. Therefore, the Food and Drug Administration (FDA) has approved Differin Gel 0.1% (adapalene), a once-daily topical retinoid gel, for over-the-counter (OTC) treatment of acne in individuals 12 years of age and older. Differin Gel 0.1% is the first retinoid to be made available OTC for the treatment of acne in the US. It contains the first new OTC active ingredient for acne treatment since the 1980s.

If you are a pro retinol user don’t be disheartened since retinol is here to stay as it is a favorite of many and there are certain benefits to the retinol legislation for EU users and lower concentrations like, same efficacy at lower price, clear percentages, good quality formulations and ingredients, bunching multiple active ingredients in one product for targeted use, and popularization of alternatives for retinol like bakuchiol, a natural plant ingredient similar to retinol.
Have you ever seen your serums changing color or creams changing texture with time?

The faulty packaging of high percentage retinol products exposes it to air and light which lead to early oxidation, only airless pump packaging can preserve it because retinol, unlike other activities, is not water soluble. Its fat soluble, quick oxidizing property can be stabilized by using lower retinol concentrations. Through the revised legislation developments in encapsulation and liposome technology aiming to improve formulations will provide better protection for sensitive ingredients such as retinol against degradation, as well as allowing a more controlled release. Beauty industry will be pushed to explore other forms of retinol like retinaldehyde and retinyl retinoate which act 11 and 8 times faster than retinol respectively.
However, retinol will still reign on the anti-aging shelf at stores but in a more informed and advanced makeover. Skin science is an ever evolving study. It would be interesting to see how brands tackle fresh ingredient insights to shape consumer perceptions in today’s social media landscape where news is just a click away, false alarms everywhere and influencer buzz raging the world of marketing. Be cautious and caring since your skin is the largest organ of your body which works day and night to sustain you. While aging gracefully is desirable, treat aging as a gift not as a flaw.









![Demna woke up and chose violence!!!
Gucci Milan Fashion Week Fall 2026, Demna’s debut Runway ✨🙇🏻♀️💋
[Demna, Gucci, Milan Fashion Week, Runway, Fashion, Italy, Let The Raven Talk]](https://lettheraventalk.com/wp-content/plugins/instagram-feed/img/placeholder.png)
